SOUTH KOREA
MFDS Registration
What is medical device in South Korea?
Medical Device Definition in regulation
The term “medical device” means an instrument, machine, apparatus, material, software, or any other similar product specified in the following subparagraphs as one used, alone or in combination, for human beings or animals: Provided, That drugs and quasi-drugs under the Pharmaceutical Affairs Act and the prosthetic limbs and aids among assistive devices for persons with disabilities under Article 65 of the Act on Welfare of Persons with Disabilities shall be excluded herefrom:
1. A product used for the purpose of diagnosing, curing, alleviating, treating, or preventing a disease;
2. A product used for the purpose of diagnosing, curing, alleviating, or correcting an injury or impairment;
3. A product used for the purpose of testing, replacing, or transforming a structure or function;
4. A product used for the control of conception.
Classification of Medical Device
There are four classifications for medical device in South Korea.
- Class I : Low risk
- Class II : Medium-Low risk
- Class III : Medium-High risk
- Class IV : High risk
Registration Procedure
Class I, Class II medical device
Most of Class I and Class II devices are reviewed under 3rd party agency. We call this as “Notification” for Class I device and “Certification” for Class II device.
Class I no need to proceed the KGMP audit but Class II or higher classification are mandatory to conduct the KGMP audit from authority.
- Class I : Notification
- Class II : Certification
Class III, Class IV medical device
Most of Class III and Class IV devices are reviewed by MFDS directly. We call this as “Permission”. Some of Class III and most of Class IV devices are required to submit the clinical evidence to support the efficacy and safety of the subject device.
Class III devices are audited by 3rd party agency for KGMP certification but Class IV device is audited by the MFDS and 3rd party auditor as mandatory.
- Class III : Permission
- Class IV : Permission
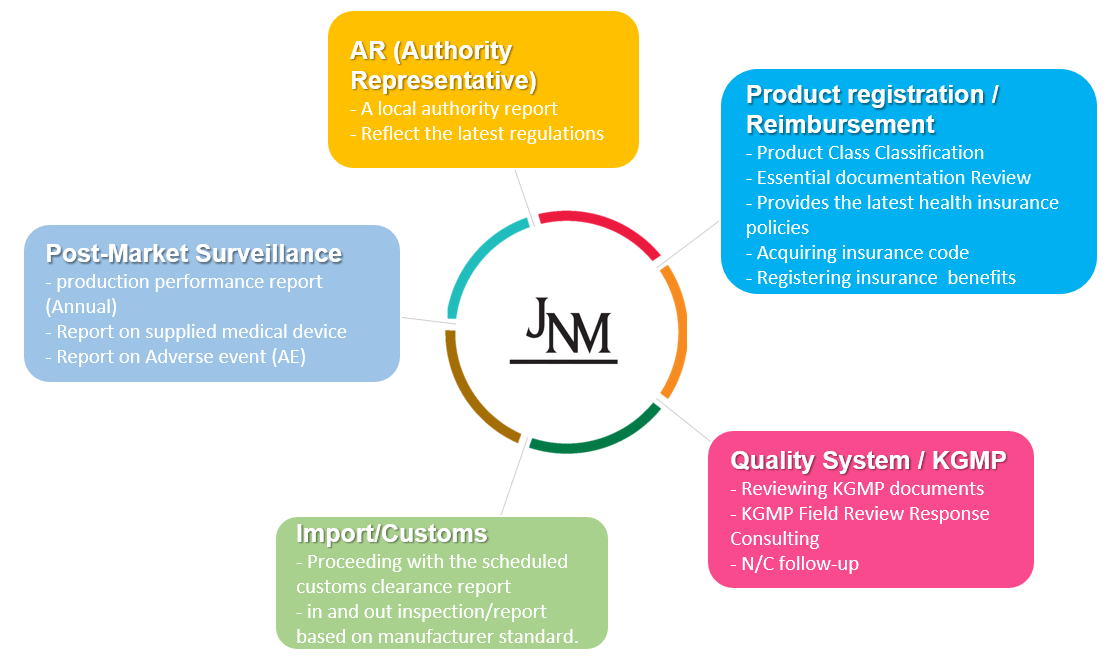
What is KGMP then?
KGMP(Korea Good Manufacturing Practice) is South Korea’s quality management system requirement mostly following the ISO13485:2016 standard.
Basically, on-site audit is required even the facility is in aboard and 3 years validity assigned after the approval.
- KGMP certification issued separately with Product registration certification.
We can provide,